
This was the beginning of further understanding leading to the atomic theory and structure that we know today.Ĭathode ray tube is a tube that contains a small amount of gasīetween two metallic plates. About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators.
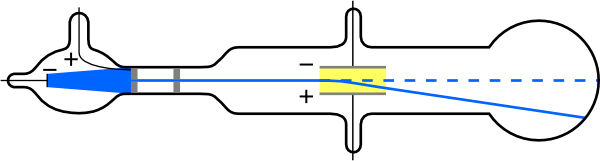
He won the 1906 Nobel prize for the discovery of electrons. He zapped atoms with electricity and observed that negatively charged particles were removed! He reasoned that atoms consisted of subatomic particles, electrons that were negatively charged particles. Thomson, led to the discovery of the negatively charged part of the atom, the electron. Thomson, a British physicist, conducted the cathode ray experiment. The cathode ray tube experiment, originally carried out by J.J. This indicated that the cathode ray was composed of negatively-charged particles. The cathode ray was deflected away from the negatively-charged electric plate and towards the positively-charged plate. Previously, atoms were known to be indivisible, but in 1897, J. To test the properties of the particles, Thomson placed two oppositely-charged electric plates around the cathode ray.


 0 kommentar(er)
0 kommentar(er)
